Reporting Adverse Clinical Privileges Actions

Figure E-2
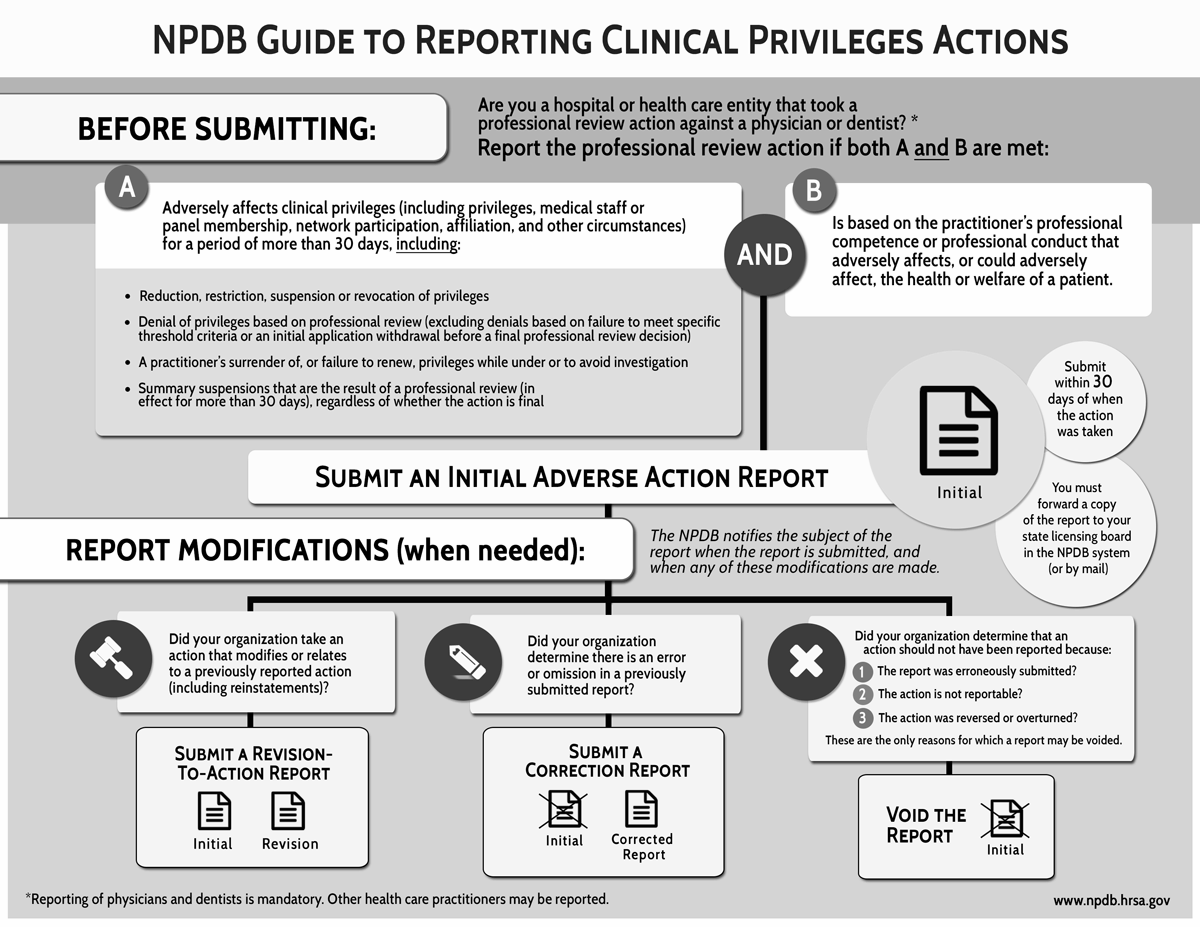
See the full infographicHospitals and other health care entities must report adverse clinical privileges actions to the NPDB that meet NPDB reporting criteria - that is, any professional review action that adversely affects the clinical privileges of a physician or dentist for a period of more than 30 days or the acceptance of the surrender of clinical privileges, or any restriction of such privileges by a physician or dentist, (1) while the physician or dentist is under investigation by a health care entity relating to possible incompetence or improper professional conduct, or (2) in return for not conducting such an investigation or proceeding. Clinical privileges include privileges, medical staff membership, and other circumstances (e.g., network participation and panel membership) in which a physician, dentist, or other health care practitioner is permitted to furnish medical care by a health care entity.
Adverse clinical privileges actions that must be reported to the NPDB are professional review actions - that is, they are based on a physician's or dentist's professional competence or professional conduct that adversely affects, or could adversely affect, the health or welfare of a patient. Generally, the entity that takes the clinical privileges action determines whether the physician's or dentist's professional competence or professional conduct adversely affects, or could adversely affect, the health or welfare of a patient. Hospitals and other health care entities must report clinical privileges actions taken against physicians and dentists when those actions meet the criteria for reportability.
In addition, hospitals and other health care entities may report - and are encouraged to report - clinical privileges actions taken against health care practitioners other than physicians and dentists when those clinical privileges actions are based on the practitioner's professional competence or professional conduct that adversely affects, or could adversely affect, the health or welfare of a patient.
Definitions and examples of these terms are provided in Chapter C: Subjects of Reports.
Table E-5 outlines reporting obligations for adverse clinical privileges actions.
| Law | Who Reports? | What is Reported? | Who is Reported? |
|---|---|---|---|
| Title IV | Hospitals Other health care entities with formal peer review |
Certain clinical privileges actions related to professional competence or conduct | Physicians and dentists Other practitioners (optional) |
Hospitals and other eligible health care entities must report:
- Professional review actions that adversely affect a physician's or dentist's clinical privileges for a period of more than 30 days
- Acceptance of a physician's or dentist's surrender or restriction of clinical privileges while under investigation for possible professional incompetence or improper professional conduct, or in return for not conducting such an investigation or not taking a professional review action that otherwise would be required to be reported to the NPDB
Actions taken against a physician's or dentist's clinical privileges include reducing, restricting, suspending, revoking, or denying privileges, and also include a health care entity's decision not to renew a physician's or dentist's privileges if that decision was based on the practitioner's professional competence or professional conduct. Clinical privileges actions are reportable once they are made final by the health care entity. However, summary suspensions lasting more than 30 days are reportable even if they are not final.
Adverse clinical privileges actions should not be reported to the NPDB unless they adversely affect the practitioner's clinical privileges for a period longer than 30 days. Matters not related to the professional competence or professional conduct of a practitioner should not be reported to the NPDB. For example, adverse actions based primarily on a practitioner's advertising practices, fee structure, salary arrangement, affiliation with other associations or health care professionals, or other competitive acts intended to solicit or retain business are excluded from NPDB reporting requirements.
Hospitals and other health care entities must report revisions to previously reported adverse clinical privileges actions. For more information, see Types of Reports.
Administrative Actions
Administrative actions that do not involve a professional review action should not be reported to the NPDB. For example: A hospital's bylaws require physicians to be board certified in their specialty. A physician's board certification expires and, as a result, the hospital automatically revokes the physician's clinical privileges through an administrative action. The revocation of clinical privileges was not a result of a professional review action and should not be reported to the NPDB.
Multiple Adverse Actions
If a single professional review action produces multiple clinical privileges actions (for example, a 12-month suspension followed by a 5-month mandatory consultation period requiring approval of a department chair before the exercise of clinical privileges), only one report, reflecting the multiple actions taken, should be submitted to the NPDB. The reporting entity may select up to five Adverse Action Classification Codes on the reporting format to describe the actions taken. Reporting entities should use the narrative description to explain any additional adverse actions imposed.
A Revision-to-Action Report must be submitted when each of the multiple actions is lifted or otherwise changed. For the example in the previous paragraph:
- If the Initial Report clearly states that the suspension is to end after 12 months, and the mandatory consultation period is to end after 5 months, and if these penalties are not changed and are fully met by the practitioner, no additional reports should be submitted
- If, after the Initial Report is submitted, the suspension period is extended to 14 months or the mandatory consultation period is shortened to 4 months, a Revision-to-Action Report must be submitted when either change is imposed
If an adverse action against the clinical privileges of a practitioner is based on multiple grounds, only a single report should be submitted to the NPDB. However, all reasons for the action should be reported and explained in the narrative description. The reporting entity may select up to four Basis for Action Codes to indicate these multiple reasons. Additional reasons should be summarized in the narrative description.
Denials or Restrictions
Denials or restrictions of clinical privileges for more than 30 days that result from professional review actions relating to the practitioner's professional competence or professional conduct that adversely affects, or could adversely affect, the health or welfare of a patient must be reported to the NPDB. This includes denials of initial applications for clinical privileges. When used by the NPDB in the context of clinical privileges actions, a "restriction" is the result of a professional review action based on clinical competence or professional conduct that leads to the inability of a practitioner to exercise his or her own independent judgment in a professional setting.
Note that a denial of clinical privileges at appointment or reappointment that occurs solely because a practitioner does not meet a health care institution's established threshold criteria for that particular privilege should not be reported to the NPDB. Such denials are not deemed the result of a professional review action relating to the practitioner's professional competence or professional conduct but are considered decisions based on eligibility. In addition, if a hospital or other health care entity retroactively changes the threshold criteria for a particular clinical privilege, a physician who does not meet the new criteria will lose previously granted clinical privileges. This loss of privileges should not be reported to the NPDB.
Examples of eligibility threshold criteria may include: (1) minimum professional liability coverage, (2) board certification, (3) geographic proximity to the hospital, and (4) performance of a minimum number of procedures prescribed for a particular clinical privilege.
Withdrawal of Applications
Voluntary withdrawal of an initial application for medical staff appointment or clinical privileges prior to a final professional review action generally should not be reported to the NPDB. However, if a practitioner applies for renewal of a medical staff appointment or clinical privileges and voluntarily withdraws that application while under investigation by the health care entity for possible professional incompetence or improper professional conduct, or in return for not conducting such an investigation or not taking a professional review action, then the withdrawal of application for renewal of clinical privileges must be reported to the NPDB. These actions must be reported regardless of whether the practitioner knew he or she was under investigation when the renewal application for medical staff appointment or clinical privileges was withdrawn. A practitioner's awareness that an investigation is being conducted is not a requirement for filing a report with the NPDB.
Nonrenewals
Nonrenewals of medical staff appointment or clinical privileges generally should not be reported to the NPDB. However, if the practitioner does not apply for renewal of medical staff appointment or clinical privileges while under investigation by the health care entity for possible professional incompetence or improper professional conduct, or in return for not conducting such an investigation or not taking a professional review action, the event is considered a surrender while under investigation and must be reported to the NPDB. These actions must be reported regardless of whether the practitioner was aware of the investigation at the time he or she failed to renew the staff appointment or clinical privileges. A practitioner's awareness that an investigation is being conducted is not a requirement for filing a report with the NPDB.
Investigations
Investigations should not be reported to the NPDB. However, a surrender of clinical privileges or failure to renew clinical privileges while under investigation or to avoid investigation must be reported.
NPDB interprets the word "investigation" expansively. It may look at a health care entity's bylaws and other documents for assistance in determining whether an investigation has started or is ongoing, but it retains the ultimate authority to determine whether an investigation exists. The NPDB considers an investigation to run from the start of an inquiry until a final decision on a clinical privileges action is reached. In other words, an investigation is not limited to a health care entity's gathering of facts or limited to the manner in which the term "investigation" is defined in a hospital's by-laws. An investigation begins as soon as the health care entity begins an inquiry and does not end until the health care entity's decision-making authority takes a final action or makes a decision to not further pursue the matter.
A routine, formal peer review process under which a health care entity evaluates, against clearly defined measures, the privilege-specific competence of all practitioners is not considered an investigation for the purposes of reporting to the NPDB. However, if a formal, targeted process is used when issues related to a specific practitioner's professional competence or conduct are identified, this is considered an investigation for the purposes of reporting to the NPDB.
A health care entity that submits a clinical privileges action based on surrender, restriction of, or failure to renew a physician's or dentist's privileges while under investigation should have evidence of an ongoing investigation at the time of surrender, or evidence of a plea bargain. The reporting entity should be able to produce evidence that an investigation was initiated prior to the surrender of clinical privileges by a practitioner. Examples of acceptable evidence may include minutes or excerpts from committee meetings, orders from hospital officials directing an investigation, or notices to practitioners of an investigation (although there is no requirement that the health care practitioner be notified or be aware of the investigation).
Guidelines for Investigations
- For NPDB reporting purposes, the term "investigation" is not controlled by how that term may be defined in a health care entity's bylaws or policies and procedures.
- The investigation must be focused on the practitioner in question.
- The investigation must concern the professional competence and/or professional conduct of the practitioner in question.
- To be considered an investigation for purposes of determining whether an activity is reportable, the activity generally should be the precursor to a professional review action.
- An investigation is considered ongoing until the health care entity's decision-making authority takes a final action or formally closes the investigation.
- A routine or general review of cases is not an investigation.
- A routine review of a particular practitioner is not an investigation.
Temporary Clinical Privileges
For the purpose of reporting to the NPDB, no distinction is made between temporary clinical privileges (including but not limited to emergency and disaster clinical privileges) and clinical privileges. If, however, temporary privileges are awarded to a physician or dentist for a specific amount of time, with no opportunity for renewal - and both the physician or dentist and the privileging party agree that the privileges are temporary - and the temporary privileges expire while the practitioner is under investigation, a report should not be submitted to the NPDB. In this scenario, there is no opportunity to renew the temporary clinical privileges, so the expiration of the temporary privileges while under investigation cannot be considered a nonrenewal or surrender of clinical privileges while under investigation.
Summary Suspensions
A summary suspension must be reported if it is:
- In effect or imposed for more than 30 days
- Based on the professional competence or professional conduct of the physician, dentist, or other health care practitioner that adversely affects, or could adversely affect, the health or welfare of a patient, and
- The result of a professional review action taken by a hospital or other health care entity
In addition, summary suspensions imposed for an indefinite length that have not lasted more than 30 days but are expected to last more than 30 days, and that are otherwise reportable, may be reported to the NPDB. If the summary suspension ultimately does not last more than 30 days, the report must be voided.
The NPDB treats summary suspensions differently from other professional review actions because the procedural rights of the practitioner are provided following the imposition of a suspension, rather than preceding it. A summary suspension is often imposed by an official (for instance, the chairman of a department) on behalf of the hospital or health care entity for the purpose of protecting patients from imminent danger. Commonly, this action is then reviewed and confirmed by a hospital committee, such as a medical executive committee (MEC), as authorized by the medical staff bylaws or other official documents (e.g., rules and procedures, standard operating procedures). Summary suspensions are considered to be effective when they go into effect, even though they may be subject to review by some committee or body of the health care entity according to the entity's bylaws or other official documents.
For purposes of reporting a summary suspension to the NPDB, if the summary suspension is confirmed by the review body, the action is considered to have taken effect when it was first imposed by the hospital official. If a summary suspension is in effect for more than 30 days before an action is taken by the authorized hospital committee or body, it must be reported to the NPDB. If the authorized hospital committee or body does not confirm the initial action or takes a different professional review action, a Revision-to-Action Report must be submitted. If the authorized hospital committee or body vacates the summary suspension, the entity must void the previous report submitted to the NPDB.
If the summary suspension subsequently is modified or revised as part of a final decision by the governing board or similar body, the health care entity must then submit a Revision-to-Action Report to supplement the Initial Report submitted to the NPDB.
If the physician, dentist, or other health care practitioner surrenders his or her clinical privileges during a summary suspension, regardless of whether the suspension has been confirmed by a hospital review body, that action must be reported to the NPDB. The action must be reported because the practitioner is surrendering the privileges either while under investigation concerning professional conduct or professional competence that did or could affect the health or welfare of a patient, or in return for not conducting an investigation concerning the same.
This reporting policy for summary suspensions is in keeping with the purpose of the NPDB, which is to protect the public from the threat of incompetent practitioners continuing to practice without disclosure or discovery of previous damaging or incompetent performance.
An action must be reported to the NPDB based on whether it satisfies NPDB reporting requirements and not based on the name affixed to the action. A suspension or restriction, whether called immediate, summary, emergency, or precautionary, typically means that a serious question has been raised and must be addressed quickly. Therefore, if a hospital or other health care entity suspects but has not confirmed a risk to an individual or individuals and imposes a suspension or restriction as immediate or precautionary, and the suspension remains in effect for more than 30 days, it must be reported to the NPDB.
Proctors
If, as a result of a professional review action related to professional competence or conduct, a proctor is required in order for a physician or dentist to proceed in freely exercising clinical privileges, and the period lasts longer than 30 days, the action must be reported to the NPDB. In other words, if, for a period lasting more than 30 days, the physician or dentist cannot perform certain procedures without proctor approval or without the proctor being present and watching the physician or dentist, the action constitutes a restriction of clinical privileges and must be reported.
However, if the proctor is not required to be present for or approve the procedures (for example, if the proctoring consists of the proctor reviewing the physician's or dentist's records or procedures after they occur), the action is not considered a restriction of clinical privileges and should not be reported to the NPDB.
Residents and Interns
Residents and interns generally should not be subjects of adverse clinical privileges actions because they are trainees in graduate health professions education programs and are not granted clinical privileges within the meaning of NPDB regulations, but they are authorized by the sponsoring institution to perform clinical duties and responsibilities within the context of their graduate educational program. However, adverse clinical privileges actions based on events occurring outside the scope of a formal graduate educational program - for example moonlighting in the intensive care unit or emergency room - must be reported to the NPDB.
Length of Restriction
Entities must report clinical privileges actions to the NPDB if they result from a professional review action and last longer than 30 days. Title IV requires "a professional review action that adversely affects the clinical privileges of a physician or dentist for longer than 30 days" to be reported (emphasis added). The NPDB has consistently interpreted "adversely affects" to mean the impact of the restriction, not the manner in which the restriction is written.
If a physician's or dentist's privileges are adversely affected for longer than 30 days, the restriction must be reported, regardless of how the health care entity writes the restriction. For example, summary suspensions must be reported if imposed or in effect for longer than 30 days. However, summary suspension reports must be voided if the reported suspension did not ultimately last longer than 30 days. As is the case with any restriction, the reportability of the action is determined by the number of days privileges are restricted.
A health care entity may choose to structure a restriction based on when a health care practitioner demonstrates clinical competence, rather than attaching a specific timeframe to the action. A significant percentage of clinical privileging actions are reported to the NPDB as "indefinite" in length, placing the responsibility on the practitioner to demonstrate to the entity that he or she no longer needs the restriction. If such an adverse action is in effect for more than 30 days, it must be reported.
With the exception of summary suspensions, which may be reported whenever a summary suspension is expected to last more than 30 days, a restriction begins at the time a physician cannot practice the full scope of his or her privileges and is reportable to the NPDB once that restriction has been in place for 31 days. For example, proctoring may or may not be reportable depending on the type of proctoring and the length of time it is in effect. If a physician or dentist cannot perform certain procedures without proctor approval or presence, this is considered a restriction on privileges. The inability to practice the full scope of privileges without a proctor's presence or approval is a restriction. Once the physician or dentist is prohibited from performing the procedure without a proctor, his or her privileges are adversely affected. The number of cases required to be proctored at the time of imposition, or the expectation that a restriction conclude in fewer than 31 days, is irrelevant for reporting purposes. The reportability of a proctoring restriction hinges on whether the restriction, in fact, is in effect for a period longer than 30 days.
Confidentiality Laws Related to Drug and Alcohol Treatment
If a clinical privileges action is taken and the practitioner enters a drug or alcohol treatment or rehabilitation program as a result, the adverse action must be reported. This is true even if the treatment is a condition of probation. However, the fact that the practitioner entered a drug or alcohol treatment facility should not be reported.
Submitting a Copy of the Report to a State Licensing Board
A copy of the report that health care entities receive after a clinical privileges action report is processed successfully by the NPDB must be provided to the appropriate state licensing board in the state in which the health care entity is located. Alternatively, NPDB reporters may elect to send an electronic version of the report to the appropriate state licensing board through the NPDB's Electronic Report Forwarding service, provided the state board has agreed to accept electronic notices of an action.
Sanctions for Failing to Report to the NPDB
A hospital or other health care entity that has substantially failed to submit adverse clinical privileges reports can lose, for 3 years, the immunity protections provided under Title IV for professional review actions it takes against physicians and dentists based on their professional competence and professional conduct.
The secretary of HHS will conduct an investigation if there is reason to believe that a health care entity has substantially failed to report required adverse actions. If the investigation reveals that the health care entity has not complied with NPDB regulations, the secretary will provide the entity with written notice describing the noncompliance. This written notice provides the entity with the opportunity to correct the noncompliance and notifies it of its right to request a hearing.
A request for a hearing must contain a statement of the material factual issues in dispute to demonstrate cause for a hearing and must be submitted to HHS within 30 days of receipt of the notice of noncompliance. These issues must be both substantive and relevant. An example of a material factual issue in dispute is a health care entity refuting HHS's claim that the health care entity failed to meet reporting requirements.
A request for a hearing will be denied if it is untimely or lacks a statement of material factual issues in dispute, or if the statement is frivolous or inconsequential. Hearings are held in the Washington, DC, metropolitan area.
If a request for a hearing is denied or if HHS determines that a health care entity has substantially failed to report information in accordance with NPDB requirements, the name of the entity will be published in the Federal Register, and the entity will lose the immunity provisions of Title IV with respect to professional review activities for a period of 3 years, commencing 30 days from the date of publication in the Federal Register.
Table E-6 provides examples of activities that should and should not be reported as clinical privileges actions.
| Based on assessment of professional competence, a proctor is assigned to a physician or dentist for a period of more than 30 days. The proctor must grant approval before the practitioner can perform procedures. | Yes |
| Based on assessment of professional competence, a proctor is assigned to supervise a physician or dentist for a period of more than 30 days, but the proctor need not be present or grant approval before medical care is provided by the practitioner. | No |
| Based on assessment of professional competence, a proctor is assigned to watch a physician's or dentist's procedures for a period of more than 30 days, and the proctor needs to be present or grant approval before medical care is provided by the practitioner. | Yes |
| Practitioners who have recently been granted clinical privileges are routinely assigned a proctor for 60 days as required by hospital policy | No |
| A physician or dentist restricts or surrenders clinical privileges; the physician or dentist is under investigation related to professional competence or professional conduct. | Yes |
| A physician or dentist restricts or surrenders clinical privileges for personal reasons; the physician or dentist is not under investigation related to professional competence or professional conduct. | No |
| A physician or dentist restricts or surrenders clinical privileges in return for not conducting an investigation related to professional competence or professional conduct. | Yes |
| A physician or dentist surrenders clinical privileges for personal reasons but is under investigation for professional competence or conduct. | Yes |
| A physician or dentist is denied medical staff appointment or clinical privileges because the health care entity has too many specialists in the practitioner's discipline | No |
| A physician's or dentist's application for medical staff appointment is denied based on a professional review action related to professional competence or professional conduct | Yes |
| A physician's or dentist's request for clinical privileges is denied or restricted for more than 30 days based upon an assessment of clinical competence as defined by the hospital. | Yes |
| A physician's or dentist's clinical privileges are suspended for reasons not related to professional competence or professional conduct. | No |
Update October 2018
The following table describes changes made to the NPDB Guidebook. Style and formatting changes made throughout the Guidebook that do not affect the substance of the text are not indicated below. References to new figures added to this edition can be found in the Table of Figures.
- Modified the section entitled "Proctors."
- Added a new section, "Length of Restriction," following the section entitled "Residents and Interns."
- Rewrote the answer to Q&A no. 9.
- Added four new Q&As following the previous Q&A no. 21 and renumbered the succeeding Q&As.
- Added a new Q&A following the previous Q&A no. 26 and renumbered the succeeding Q&As.
- Added a new Q&A following the previous Q&A no. 40 and renumbered the succeeding Q&As.
- Added a new Q&A following the previous Q&A no. 42.
Sections Updated
 An official website of the United States government.
An official website of the United States government.

